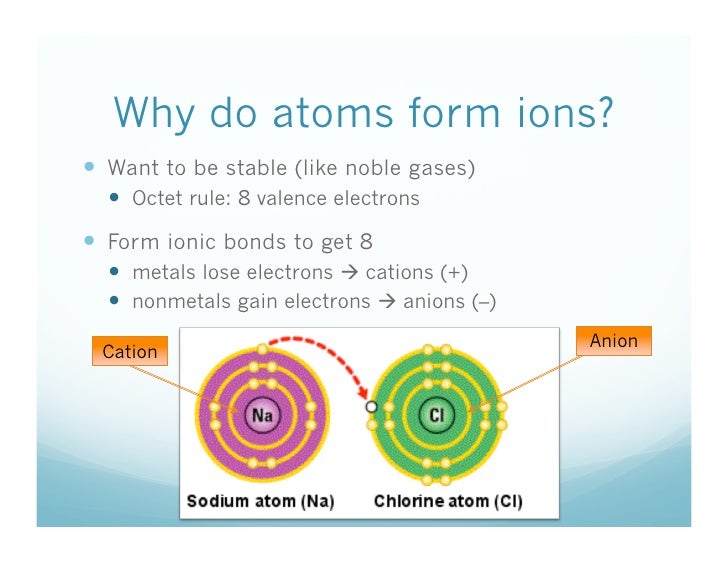
Atoms start out electrically neutral because they have the same number of negatively charged electrons and positively protons. However, An ion is an atom that has gained or lost one or more electrons. Therefore, it has a negative or positive charge.
For example, Sodium. A sodium atom has one electron in the outer shell. A chlorine atom seven electrons in the outer shell. A sodium atom loses an electron to a chlorine atom. The sodium atom becomes a positive sodium ion.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.